Aldeyra Therapeutics Announces Advancement of New RASP Modulators and Recent Preclinical Data in Obesity at 2024 Investor Roundtable
Live Webcast Scheduled to Begin at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240620180926/en/
(Graphic: Aldeyra)
Pipeline Updates
- Following positive biomarker results in adults, including near-normalization of lipid profiles, ADX-629, a first-in-class orally administered investigational RASP modulator, advanced to the pediatric cohort of the Phase 2 clinical trial in Sjögren-Larsson Syndrome, a rare inborn error of metabolism caused by mutations in fatty aldehyde dehydrogenase and characterized by cognitive dysfunction and skin disorders. Top-line results from approximately five pediatric patients are expected in 2025.
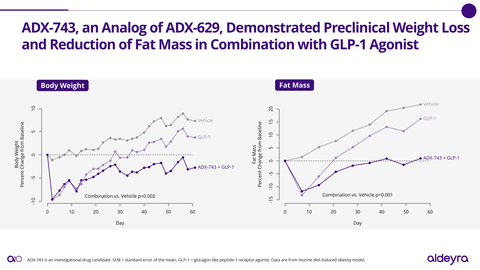
- Based on new results in a preclinical model of obesity, novel RASP modulator ADX-743 was advanced to Investigational New Drug (IND)-enabling studies. ADX-743 is an analog of ADX-629, which has demonstrated lowering of triglycerides and fatty acids in Phase 1 and Phase 2 clinical trials. Pending additional results, Aldeyra expects to submit an IND application for ADX-743 or an alternative RASP modulator for obesity or hypertriglyceridemia in 2025.
- Based in part on superior preclinical results in atopic dermatitis and unprecedented exposure in preclinical RASP modulator pharmacokinetic models, ADX-248 will be advanced in lieu of ADX-246 to Phase 1/2 clinical testing in atopic dermatitis. Phase 1 clinical testing of ADX-248 is expected to begin in the second half of 2024.
- Novel RASP modulator ADX-631 has initiated preclinical testing in models of retinal disease. ADX-631 is an analog of ADX-103, which previously demonstrated activity in animal models of the dry form of age-related macular degeneration (dry AMD), retinal inflammation, and diabetic macular edema. Aldeyra expects to submit an IND application for ADX-631 or an alternative RASP modulator for the treatment of dry AMD or geographic atrophy in the first half of 2025.
“Consistent with the new data presented at our Research and Development Day in April of this year, we continue to expand our novel RASP modulator pipeline with the discovery and advancement of new RASP modulators for the treatment of inflammatory and metabolic diseases,” stated
Investor Roundtable Webcast Information
The 2024 Aldeyra Therapeutics Investor Roundtable will take place at
About Aldeyra
Safe Harbor Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Aldeyra’s future expectations, plans, and prospects, including without limitation statements regarding: the goals, opportunity, and potential for Aldeyra’s product candidates; the outcome and expected timing and the results of Aldeyra’s planned preclinical and clinical trials, including planned and ongoing trials; the outcome and timing of the FDA’s review, acceptance and/or approval of IND submissions for Aldeyra’s product candidates. Aldeyra intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” "could," “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. All of Aldeyra's development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, funding, and other factors that could delay the initiation, enrollment, or completion of clinical trials. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of enrollment, commencement and completion of Aldeyra's clinical trials, the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; delay in or failure to obtain regulatory approval of Aldeyra's product candidates, including as a result of the FDA not accepting Aldeyra’s regulatory filings, issuing a complete response letter, or requiring additional clinical trials or data prior to review or approval of such filings or in connection with resubmissions of such filings; the ability to maintain regulatory approval of Aldeyra's product candidates, and the labeling for any approved products; the risk that prior results, such as signals of safety, activity, or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Aldeyra's product candidates in clinical trials focused on the same or different indications; the scope, progress, expansion, and costs of developing and commercializing Aldeyra's product candidates; uncertainty as to Aldeyra’s ability to commercialize (alone or with others) and obtain reimbursement for Aldeyra's product candidates following regulatory approval, if any; the size and growth of the potential markets and pricing for Aldeyra's product candidates and the ability to serve those markets; Aldeyra's expectations regarding Aldeyra's expenses and future revenue, the timing of future revenue, the sufficiency or use of Aldeyra's cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra's product candidates; Aldeyra's expectations regarding competition; Aldeyra's anticipated growth strategies; Aldeyra's ability to attract or retain key personnel; Aldeyra’s commercialization, marketing and manufacturing capabilities and strategy; Aldeyra's ability to establish and maintain development partnerships; Aldeyra’s ability to successfully integrate acquisitions into its business; Aldeyra's expectations regarding federal, state, and foreign regulatory requirements; political, economic, legal, social, and health risks, public health measures, and war or other military actions, that may affect Aldeyra’s business or the global economy; regulatory developments in
In addition to the risks described above and in Aldeyra's other filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240620180926/en/
Investor & Media Contact:
Tel: (917) 618-2651
investorrelations@aldeyra.com
Source: