Aldeyra Therapeutics Highlights Recent Preclinical Data in Obesity, Atopic Dermatitis, Pain, and Alcoholic Hepatitis, and Announces Planned Pivotal Clinical Trial in Retinitis Pigmentosa, at 2024 Research & Development Day
Live Webcast Scheduled to Begin at
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240425445928/en/
(Graphic:
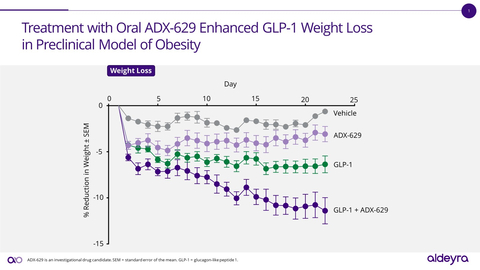
Aldeyra will present new preclinical data from investigational RASP modulators in animal models for obesity, atopic dermatitis, inflammatory pain, and alcoholic hepatitis. In the diet-induced model of obesity, ADX-629 decreased weight and fat mass alone and in combination with a GLP-1 agonist. In the oxazolone model of atopic dermatitis, RASP modulators ADX-629, ADX-246, and ADX-248 demonstrated activity in reducing skin thickness and erosion, and in reducing spleen to body weight ratio. In the carrageenan model of inflammatory pain, ADX-246 increased tolerance to mechanical and thermal pain, and decreased joint swelling. Consistent with previously released data from ADX-629 in a model of alcoholic hepatitis, ADX-246 reduced levels of fibrosis and fat in liver.
“The new data released today support the expansion of our novel RASP platform into clinical indications that may include fat-mass-targeted weight loss and inflammatory pain, highlighting the breadth of potential product candidate opportunities afforded by modulating RASP levels,” stated
Based on recent discussions with the
“Due to loss of vision and dramatic impact on quality of life, retinitis pigmentosa remains a highly significant unmet medical need in retinal disease,” stated
Research & Development Day Webcast Information
Aldeyra’s Research & Development Day will take place from
About Aldeyra
Safe Harbor Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Aldeyra’s future expectations, plans, and prospects, including without limitation statements regarding: the goals, opportunity, and potential for reproxalap, ADX-2191, and other product candidates; the outcome and expected timing and the results of Aldeyra’s planned clinical trials, including planned and ongoing clinical trials for reproxalap and ADX-2191; the outcome and timing of the FDA’s review, acceptance and/or approval of a NDA resubmission for reproxalap and the adequacy of the data included in the original NDA; and the potential NDA resubmission. Aldeyra intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” "could," “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. All of Aldeyra's development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, funding, and other factors that could delay the initiation, enrollment, or completion of clinical trials. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of enrollment, commencement and completion of Aldeyra's clinical trials, the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; delay in or failure to obtain regulatory approval of Aldeyra's product candidates, including as a result of the FDA not accepting Aldeyra’s regulatory filings, issuing a complete response letter, or requiring additional clinical trials or data prior to review or approval of such filings or in connection with resubmissions of such filings; the ability to maintain regulatory approval of Aldeyra's product candidates, and the labeling for any approved products; the risk that prior results, such as signals of safety, activity, or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Aldeyra's product candidates in clinical trials focused on the same or different indications; the scope, progress, expansion, and costs of developing and commercializing Aldeyra's product candidates; uncertainty as to Aldeyra’s ability to commercialize (alone or with others) and obtain reimbursement for Aldeyra's product candidates following regulatory approval, if any; the size and growth of the potential markets and pricing for Aldeyra's product candidates and the ability to serve those markets; Aldeyra's expectations regarding Aldeyra's expenses and future revenue, the timing of future revenue, the sufficiency or use of Aldeyra's cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra's product candidates; Aldeyra's expectations regarding competition; Aldeyra's anticipated growth strategies; Aldeyra's ability to attract or retain key personnel; Aldeyra’s commercialization, marketing and manufacturing capabilities and strategy; Aldeyra's ability to establish and maintain development partnerships; Aldeyra’s ability to successfully integrate acquisitions into its business; Aldeyra's expectations regarding federal, state, and foreign regulatory requirements; political, economic, legal, social, and health risks, public health measures, and war or other military actions, that may affect Aldeyra’s business or the global economy; regulatory developments in
In addition to the risks described above and in Aldeyra's other filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240425445928/en/
Investor & Media:
Tel: (917) 618-2651
investorrelations@aldeyra.com
Source: